Today, chronic pain affects approximately 11% to 40% of North Americans, significantly diminishing their quality of life. It is often linked with mood disorders and represents a costly public health issue. Our failure to effectively manage chronic pain has contributed to the opioid crisis. A deeper understanding of the mechanisms behind chronic pain could lead to the development of new, non-addictive painkillers, which are crucial in combating the opioid crisis. Although pain is triggered by the activation of neuronal pathways, the role of immune cells and their secreted mediators in influencing neuronal physiology is substantial. The connection between the immune system and pain dates back to ancient Greece, where pain was considered a primary indicator of inflammation. This is further supported in modern times by the pain-relieving effects of anti-inflammatory medications such as ibuprofen, aspirin, ketoprofen, and diclofenac. Although it is well established that the immune system contributes to the development of chronic pain, we challenged the dogma that immune mediators are solely causing pain. Research conducted by us and others indicates that the immune system also plays a critical role in alleviating pain. Notably, we were among the pioneers to demonstrate the crucial role of T cells, a type of white blood cell, in the resolution of pain.
In our lab, we are dedicated to exploring how peripheral sensory neurons communicate with immune cells and how this interaction affects both the onset and resolution of pain. Understanding the dialogue between the immune and nervous systems may be key to developing effective treatments for chronic pain.
Beyond advancing our knowledge of pain's neuroimmunology, we are also committed to promoting diversity in STEM research and education. We believe that embracing inclusion and diversity sparks innovation, enabling us to address complex challenges more effectively. Our efforts are aimed at increasing the presence of underrepresented minorities within the scientific community.
Geoffroy is an Assistant Professor in the Department of Physiology and the Neuroscience program at Michigan State University. Geoffroy is interested in the contribution of Neuro-Immune interactions to chronic pain development and resolution. He received his PhD degree from the Pasteur Institute / University of Lille in France. He moved to the US to perform his postdoctoral training at MD Anderson Cancer Center under the mentorship of Drs. Hui-Lin Pan, Jean-Pierre Issa, Cobi Heijnen, Robert Danzter, and Annemieke Kavelaars. In addition to research, Geoffroy likes exploring nature, spending family time, and read about European middle ages.

Dr. Joseph Folger got his bachelors and PhD from Cornell University. He then spent 13 years working for Dr. George Smith in MSU’s Department of Animal Science. He worked on numerous projects in female bovine reproduction, primarily focused on the question of what makes a good egg a good egg. Joseph started working for Dr. Laumet in January 2020 and has been learning behavioral assays and an entirely new field of research into chronic pain. Joseph currently manages the lab and the mouse colony and assists with everyone’s projects. For fun, He likes to read, play video games and spend time with my wife, daughter and pets.

Dr Hui Xu is a research faculty member. She received her PhD from Kagawa Medical University in Japan. She worked in the Department of Pharmacology and Toxicology at Michigan State University for 25 years. Her research has focused on the autonomic nervous system, molecular signaling, and receptor/ion channel interactions in the cardiovascular and gastrointestinal system, and how these factors contribute to human disease, such as septic shock, obesity/metabolic disorders, hypertension, renal inflammation/remodeling and fibrosis, as well as Alzheimer’s and Parkinson’s associated gastrointestinal dysmotility. She joined Dr. Laumet lab in March 2025.

Sabrina received a PhD in immunology at the University of Rio de Janeiro in Brazil. She joined the Laumet in February 2022 to investigate the role of immune cells on pain and the contribution of sensory neurons to immunoregulation. In addition to Research, Sabrina mentors undergraduate and junior graduate students in the lab.

Jaewon received her B.S. in biotechnology at Korea University. She started her Ph.D. in Cell and Molecular biology at Michigan State University in 2021. Her research interest is neuroimmune interaction underlying pain. She is studying how immune cells can dampen or aggravate the pain sensation by modifying the neuron as well as the inflammatory environment. In her free time, Jaewon enjoys playing piano, listening to classical music, and watching cute cat videos.

Aaryn received her B.S. in biology from Spelman College. She came to MSU to pursue her Ph.D. in Molecular, Cellular, and Integrative Physiology and joined the lab in January 2024. She is interested in researching how neurons mediate pain via the release of extracellular vesicles. In her free time, Aaryn enjoys reading, painting, and baking.

Alex graduated from Randolph-Macon College with a B.S. in Behavioral Neuroscience and Spanish. She is pursuing her Ph.D. in Neuroscience and joined the Laumet lab in January of 2024 with an interest in investigating the physiological mechanisms that influence migraine pain. In her free time, Alex enjoys cooking, travelling, and hanging out with her cat, Caboose.

Nithila Kannan is a second-year medical student at Michigan State University College of Osteopathic Medicine and joined the Laumet Pain Lab in Spring 2025. She earned her B.S. in Human Biology from Michigan State University.
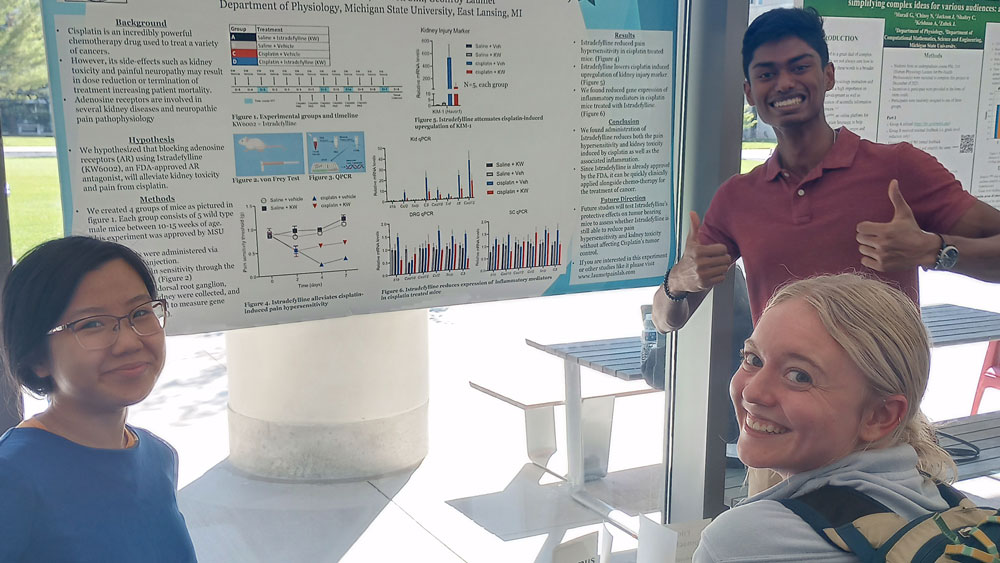
Nithila is actively engaged in clinical research spanning neurology, radiology, and endocrinology. In the Laumet Pain Lab, she investigates biomarkers and neuronal mechanisms underlying cisplatin-induced kidney injury and neuropathy.
She is passionate about mentorship, community outreach, and leveraging technology to improve patient care — interests that continue to shape her goal of pursuing a career in neuroradiology. Outside of academics, Nithila enjoys Indian classical dance, singing, exploring local coffee shops, and spending quality time with friends and family

Gayoung earned a B.S. in Biomolecular Science with a minor in Medical Anthropology from the University of Michigan–Ann Arbor in 2023. As a DO/PhD student at MSU in the Molecular, Cellular, and Integrative Physiology program, she balances laboratory research and medical school over an eight-year program. In January 2025, she joined the Laumet lab to study the role of TRPV1+ neurons in cisplatin-induced nephrotoxicity. In her free time, Gayoung enjoys reading the Bible, playing the piano and violin, cooking, and spending time with dogs.
Sophie received her B.S. degree from the University of Lille in France. She assists with the management of the mouse colony, ordering, behavioral testing, injections, tissue collections, and lab organization. Sophie enjoys running, baking and cooking.

Dashiell Jones is an undergraduate studying neuroscience through the College of Natural Science and the Honors College. He joined the lab through a professorial assistantship. He studies IL10's role in pain resolution. After graduation, he plans to attend medical school. Dash is a member of the Big10 champion MSU water polo team and a long-distance freestyle swimmer on the MSU swim team.

Rhianna is an undergraduate student at MSU studying Physiology and Neuroscience. After undergrad, she plans to obtain a higher degree and contribute to research on neurological diseases. In her free time, she loves to crochet, make jewelry, dance, and is a member of the Spartan Marching Band

Gwen is an undergraduate student studying Neuroscience through the College of Natural Science. She assists with various projects in the lab including neuroimmune interactions underlying pain, and the pain response in migraines. In her free time, she runs MSU's Cycling Club, sends mountain bike trails, plays flute or piano, or reads.

















Mast cell-derived chymases are essential for the resolution of inflammatory pain in mice. de Souza S, Laumet S, Sim J, Inyang KE, Folger JK, Hua H, Moeser AJ, Laumet G. Pain. 2025 Mar 4. doi: 10.1097/j.pain.0000000000003565. Epub ahead of print. PMID: 40035664.
Neurotensin-expressing lateral hypothalamic neurons alleviate neuropathic and inflammatory pain via neurotensin receptor signaling. Khan R, Lee B, Inyang K, Bemis H, Bugescu R, Laumet G, Leinninger G. Neurobiol Pain. 2024 Oct 19;16:100172. doi: 10.1016/j.ynpai.2024.100172. PMID: 39524478; PMCID: PMC11550133.
Upregulation of delta opioid receptor by meningeal interleukin-10 prevents relapsing pain. Inyang KE, Sim J, Clark KB, Geron M, Monahan K, Evans C, O'Connell P, Laumet S, Peng B, Ma J, Heijnen CJ, Dantzer R, Scherrer G, Kavelaars A, Bernard M, Aldhamen YA, Folger JK, Bavencoffe A, Laumet G. Brain Behav Immun. 2025 Jan;123:399-410. doi: 10.1016/j.bbi.2024.09.031. Epub 2024 Sep 29. PMID: 39349285; PMCID: PMC11624093.
Hungry for relief: Potential for neurotensin to address comorbid obesity and pain. Khan R, Laumet G, Leinninger GM. Appetite. 2024 Sep 1;200:107540. doi: 10.1016/j.appet.2024.107540. Epub 2024 Jun 7. PMID: 38852785.
Massage-like stroking produces analgesia in mice. Waarala ZMS, Comins L, Laumet S, Folger JK, Laumet G. Neurobiol Pain. 2023 Dec 24;15:100149. doi: 10.1016/j.ynpai.2023.100149. PMID: 38226332; PMCID: PMC10788302.
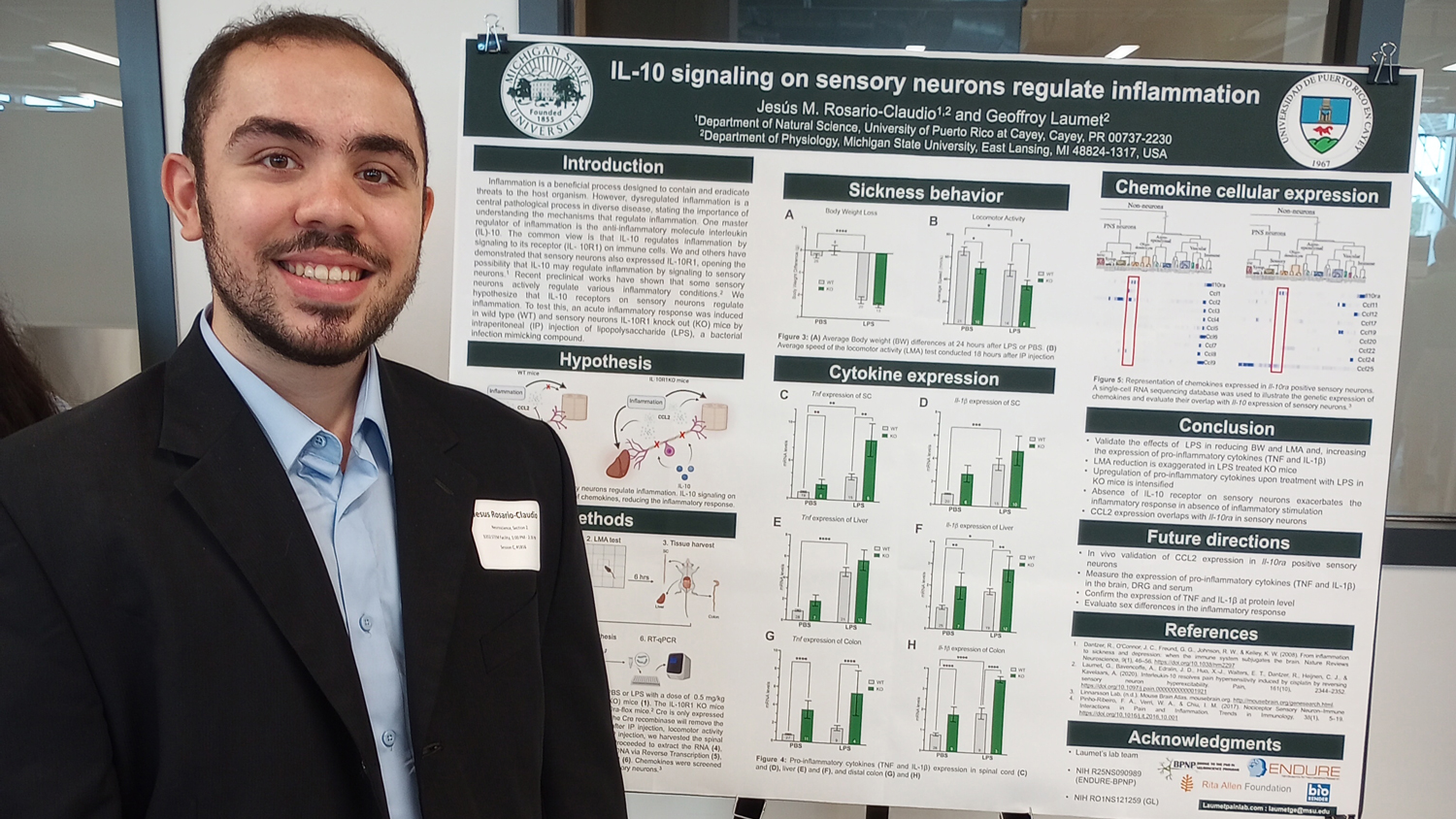
Interleukin-10 signaling in somatosensory neurons controls CCL2 release and inflammatory response. de Souza S, Rosario Claudio J, Sim J, Inyang KE, Dagenais A, Monahan K, Lee B, Ramakrishnan H, Parmar V, Geron M, Scherrer G, Folger JK, Laumet G. Brain Behav Immun. 2024 Feb;116:193-202. doi: 10.1016/j.bbi.2023.12.013. Epub 2023 Dec 9. PMID: 38081433; PMCID: PMC10843623.
. Peripheral somatosensory neurons listen and orchestrate the immune response. Laumet G. J Allergy Clin Immunol. 2023 Nov 22:S0091-6749(23)01479-3. doi: 10.1016/j.jaci.2023.11.014. Epub ahead of print. PMID: 37995857.
A peptidomimetic modulator of the CaV2.2 N-type calcium channel for chronic pain. Gomez K, Santiago U, Nelson TS, Allen HN, Calderon-Rivera A, Hestehave S, Rodríguez Palma EJ, Zhou Y, Duran P, Loya-Lopez S, Zhu E, Kumar U, Shields R, Koseli E, McKiver B, Giuvelis D, Zuo W, Inyang KE, Dorame A, Chefdeville A, Ran D, Perez-Miller S, Lu Y, Liu X, Handoko, Arora PS, Patek M, Moutal A, Khanna M, Hu H, Laumet G, King T, Wang J, Damaj MI, Korczeniewska OA, Camacho CJ, Khanna R. Proc Natl Acad Sci U S A. 2023 Nov 21;120(47):e2305215120. doi: 10.1073/pnas.2305215120. Epub 2023 Nov 16. PMID: 37972067; PMCID: PMC10666126.
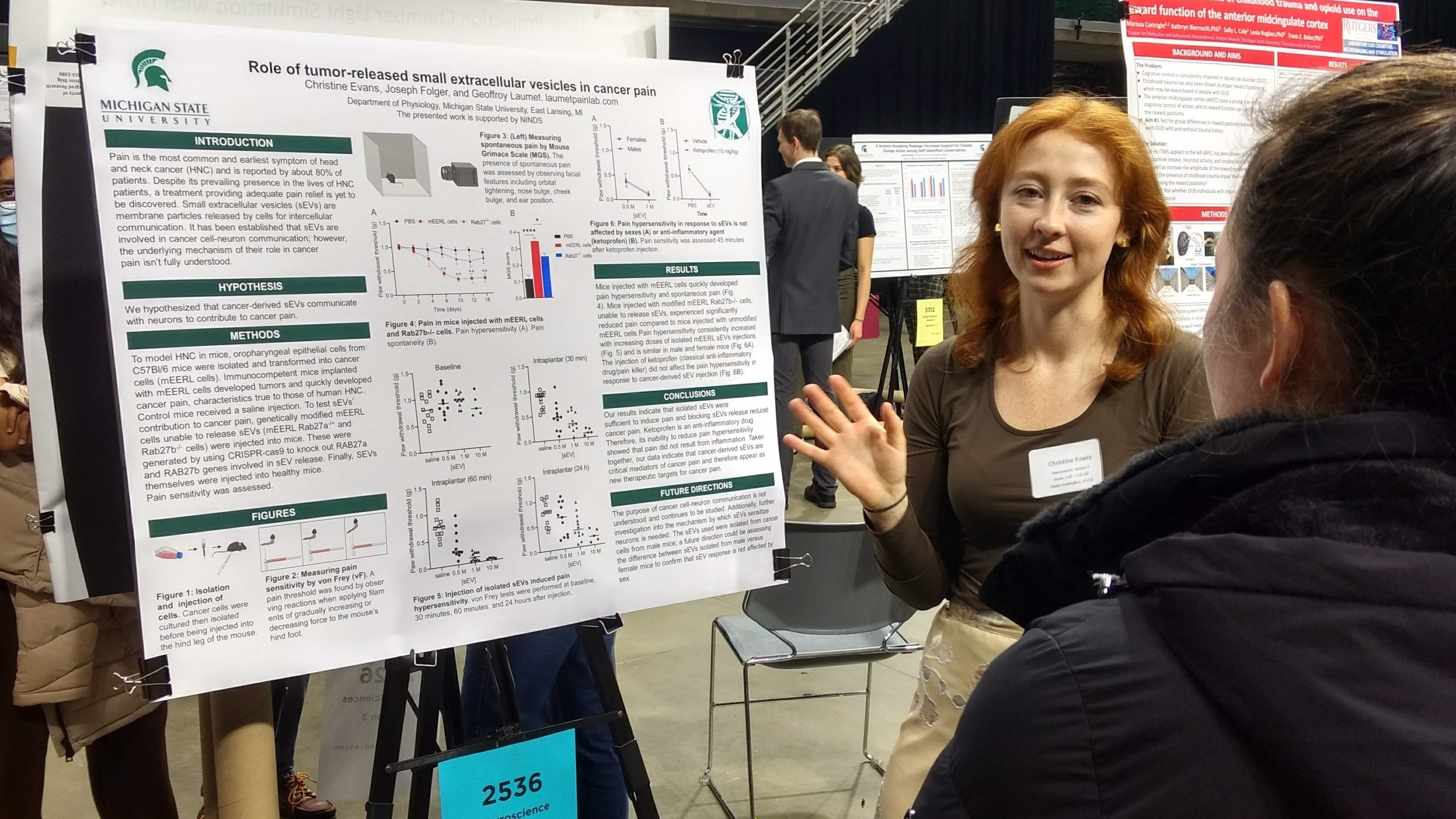
HPV+ head and neck cancer-derived small extracellular vesicles communicate with TRPV1+ neurons to mediate cancer pain. Inyang KE, Evans CM, Heussner M, Petroff M, Reimers M, Vermeer PD, Tykocki N, Folger JK, Laumet G Pain. 2023 Sep 6. doi: 10.1097/j.pain.0000000000003045. Epub ahead of print. PMID: 37678566.
Istradefylline protects from cisplatin-induced nephrotoxicity and peripheral neuropathy while preserving cisplatin antitumor effects. Dewaeles E, Carvalho K, Fellah S, Sim J, Boukrout N, Caillierez R, Ramakrishnan H, Van der Hauwaert C, Vijaya Shankara J, Martin N, Massri N, Launay A, Folger JK, de Schutter C, Larrue R, Loison I, Goujon M, Jung M, Le Gras S, Gomez-Murcia V, Faivre E, Lemaire J, Garat A, Beauval N, Maboudou P, Gnemmi V, Gibier JB, Buée L, Abbadie C, Glowacki F, Pottier N, Perrais M, Cunha RA, Annicotte JS, Laumet G, Blum D, Cauffiez C. J Clin Invest. 2022 Nov 15;132(22):e152924. doi: 10.1172/JCI152924.
T-cell activation state differentially contributes to neuropsychiatric complications in women with HIV. Williams DW, Flores BR, Xu Y, Wang Y, Yu D, Peters BA, Adedimeji A, Wilson TE, Merenstein D, Tien PC, Cohen MH, Weber KM, Adimora AA, Ofotokun I, Fischl M, Turan J, Turan B, Laumet G, Landay AL, Dastgheyb RM, Gange SJ, Weiser SD, Rubin LH. Brain Behav Immun Health. 2022 Aug 29;25:100498. doi: 10.1016/j.bbih.2022.100498. eCollection 2022 Nov.
Infiltration of peripheral immune cells into the olfactory bulb in a mouse model of acute nasal inflammation. Asano H, Hasegawa-Ishii S, Arae K, Obara A, Laumet G, Dantzer R, Shimada A. J Neuroimmunol. 2022 Jul 15;368:577897. doi: 10.1016/j.jneuroim.2022.577897. Epub 2022 May 23.
CD8+ T cell-derived IL-13 increases macrophage IL-10 to resolve neuropathic pain. Singh SK, Krukowski K, Laumet GO, Weis D, Alexander JF, Heijnen CJ, Kavelaars A. JCI Insight. 2022 Mar 8;7(5):e154194. doi: 10.1172/jci.insight.154194.
Prdm12 modulates pain-related behavior by remodeling gene expression in mature nociceptors. Latragna A, Sabaté San José A, Tsimpos P, Vermeiren S, Gualdani R, Chakrabarti S, Callejo G, Desiderio S, Shomroni O, Sitte M, Kricha S, Luypaert M, Vanhollebeke B, Laumet G, Salinas G, Smith ESJ, Ris L, Bellefroid EJ. Pain. 2022 Aug 1;163(8):e927-e941. doi: 10.1097/j.pain.0000000000002536. Epub 2021 Dec 24.
Effects of placebo administration on immune mechanisms and relationships with central endogenous opioid neurotransmission. Prossin A, Koch A, Campbell P, Laumet G, Stohler CS, Dantzer R, Zubieta JK. Mol Psychiatry. 2022 Feb;27(2):831-839. doi: 10.1038/s41380-021-01365-x. Epub 2021 Oct 29.
 Interdisciplinary Science & Technology building (ISTB)
Interdisciplinary Science & Technology building (ISTB)
Please inquire with Dr. Laumet about current opportunities.
Dr. Laumet is a faculty member in the following training programs. Students in these programs can inquire about rotations in the lab.